Optimize Clinical Trials: Framework for Patient Insights Translation
The value of patient insights falls flat if they are not actionable. Conversely, when properly captured and translated, patient insights will positively impact the entire clinical development process.
Companies can use data on structural barriers to participation, hurdles to overcome, and patient journeys to minimize logistical barriers in each stage of trial planning and rollout by ensuring there are processes in place for these insights to be implemented. One of the main enablers is a structured organizational process that ensures every team is equipped to understand and use the data.
Each organization will use a different methodology for setting these insights into action. The example below provides a conceptual framework, and can be adapted to meet the broader needs of the organization.
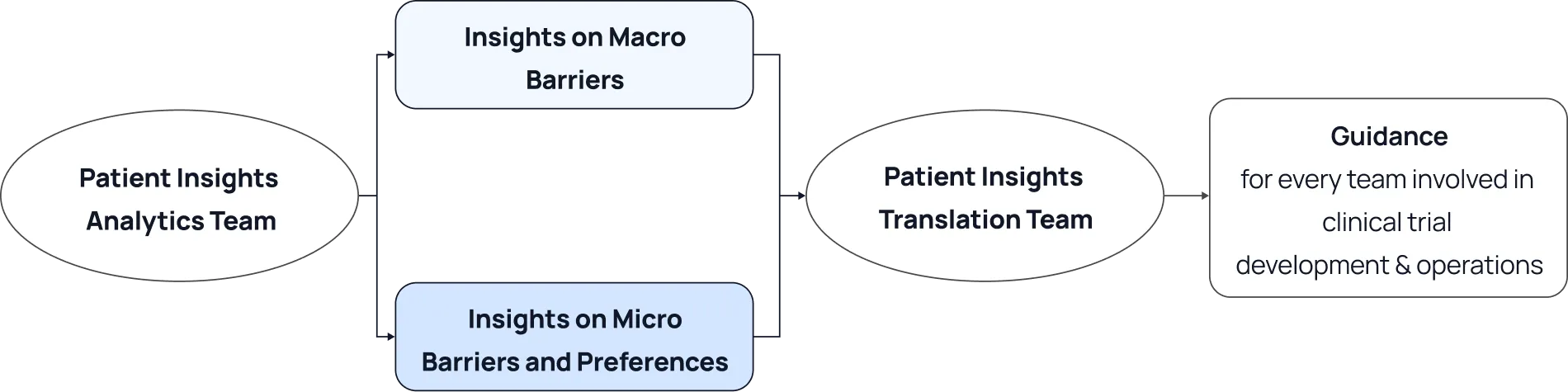
The core concept in creating organizational processes for the translation of patient insights is to structure teams and establish protocols to facilitate efficient flow of insights from collection to implementation at every point of new solution development.
Discover the Next Generation of Patient Recruitment
Get access to our white paper 'Blueprint for Modern Patient Recruitment' and discover the key strategies to enhance your clinical trial outcomes.
The first crucial team is a Patient Insight Analytics Team.
This team should collect data on:
- Therapeutic area-specific structural barriers to research participation.
- Associated patient hurdles to research participation.
- Patient journeys from trial awareness to decision making (informed consent).
- Stated and revealed patient preferences, captured before and throughout the trial.
The team should hire specialists in both qualitative and quantitative insights, with proven abilities to communicate and disseminate these insights to cross-functional teams.
The next crucial team will be devoted to patient insight translation.
This team is responsible for taking insights from the analytics team and disseminating these insights to the trial rollout team via guidance or SOPs. The team should have the necessary authority to create company-wide or portfolio-wide guidance, with the expectation that all team members will implement these materials into their daily workflows.
Ideally, this team should be led by a VP or Director with a dedicated staff for translating data into cross-team guidance. The team should also comprise monitors to ensure that guidelines are being met and that these guidelines are translating into positive forward strides in patient recruitment. The monitoring team can work with the data analytics team to review KPIs.
The trial rollout team is the final and most important team in the process.
This team is responsible for implementing the patient insights in trial design and execution. The trial rollout team should include representatives from various functions, such as clinical operations, medical affairs, regulatory affairs, and marketing. The trial rollout team should also collaborate with external stakeholders, such as investigators, site staff, and patient advocacy groups, to ensure the trial is aligned with patient needs and preferences (i.e., patient centric).
The following diagram illustrates the proposed framework for the organizational design to support the translation of patient insights into action:
The benefits of this framework are manifold.
It ensures that patient insights are not lost or ignored in the processes of trial development and execution. The added benefit here is that large investments in data and insight collection are fully leveraged and implemented in real actions rather than wasted.
It also enhances the quality and efficiency of the trial by reducing the risk of patient dropouts, increasing patient satisfaction, and improving patient outcomes. Consequently, time to market decreases and fewer protocol amendments and new site start-ups are needed, drastically improving financial outcomes.
Effective organizational design supporting the translation of patient insights is a key factor in maximizing the value of patient insights for clinical trials.
By creating teams and/or processes that facilitate the flow of patient insights from collection to implementation, companies can optimize their trial performance and deliver solutions that truly meet the real needs of patients.