Clinical Trial Phases
Introduction
A clinical trial is a type of research that helps developers of drugs, biologicals, and medical devices to ensure the safety of their products by testing and evaluating their effects on human volunteers. It helps answer several questions regarding the reliability and authenticity of a drug or treatment; Does the treatment work? Is it more effective than existing treatments? Does it have side effects? Or how this change/addition improves an existing treatment? Clinical trials are designed and reviewed and need to be approved by the FDA before starting. Each trial is conducted through a comprehensive protocol and consists of multiple clinical trial phases.
Each clinical trial phase is designed to answer different questions, ensure the product's safety for the general population, and safeguard the volunteers' information. In this guide, we will briefly discuss each clinical trial phase and the preclinical studies and major FDA interventions conducted to paint the whole picture of a drug's journey through the clinical research process.
Overview of the Stages of Clinical Research
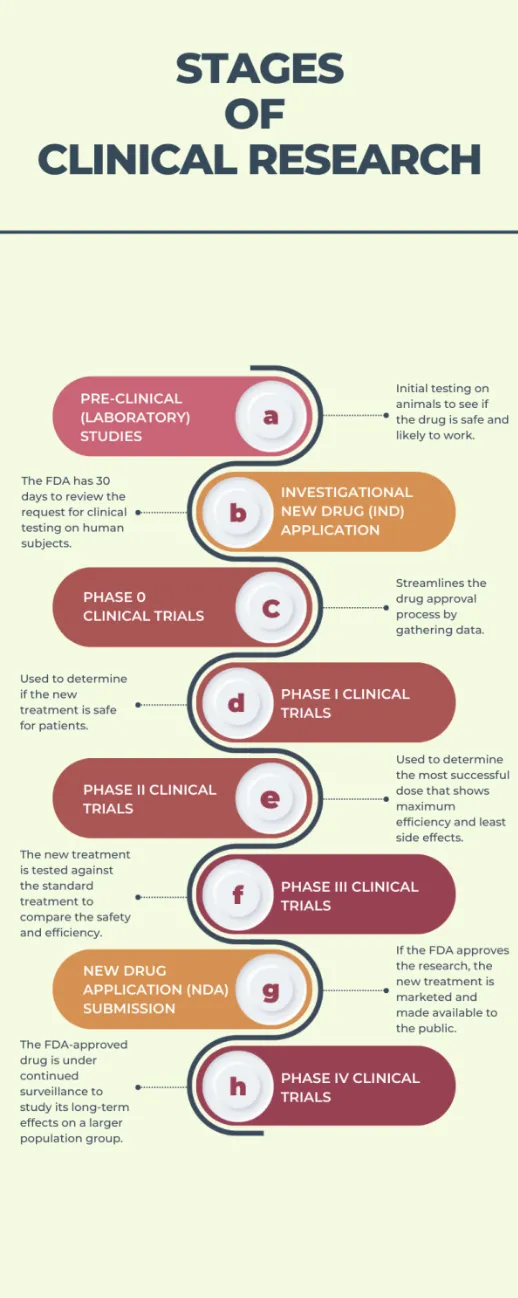
Before we dive deep into the phases of clinical trials, let's first understand what clinical research is and the basics of all phases' trial definitions.
Clinical research is a comprehensive medical study that studies the effectiveness and safety of new drugs, treatments, and devices for patient care. There are two types of clinical research, observational studies, and clinical trials. In an observational study, investigators study and analyze the health patterns in group participants through medical data or biological samples, as required by a protocol or research plan. It differs from clinical trials as participants might receive procedures as part of their medical routine but are not assigned to specific interventions, like in clinical trials.
A clinical research study goes through multiple phases before being approved by the FDA. Below is a summarized overview and breakdown of the stages of clinical research.
A. Pre-clinical (laboratory) studies
Research is only processed to a clinical trial after pre-clinical studies show that the new treatment or drug is safe and likely to work. It includes cell and animal studies and provides useful information, but only partially accurate for human subjects. Thus, further research is required.
B. Investigational New Drug (IND) application: FDA has 30 days for review
Before starting a clinical trial, an investigational new drug (IND) application or request must be filed with the FDA so that the research is approved and a study on human volunteers can be conducted. Following are the actions the FDA can take after receiving complete information about the pre-clinical studies:
- Approval → permission to begin clinical trials on human volunteers
- Disapproval ("Hold") → delay or stop the investigation, potentially requiring modifications to the protocol before continuing
C. Phase 0 clinical trials
The phase 0 clinical trial helps save time, money, and resources by providing a faster way to streamline the drug approval process. In this phase, you can gather data to see whether the drug will work on humans.
D. Phase I clinical trials
This phase includes 20 to 100 patients and is used to determine if the new treatment is safe and the best way to give the new treatment to patients.
E. Phase II clinical trials
If a treatment is found to be safe in phase I clinical trials, it proceeds to phase II. In this phase, it is determined if a treatment is effective for certain types of cancer or the most successful dose that shows maximum efficiency and least side effects, depending on what is being studied.
F. Phase III clinical trials
In phase III, also known as pivotal studies, the new treatment is tested against the standard treatment to compare the safety and efficiency. The trial consists of two groups, control (group gets the standard treatment) and study ((group gets the new treatment).
If a treatment or drug is more effective than the standard treatment, an NDA is submitted to the Food and Drug Administration for approval. According to this, a pivotal study is further categorized into two parts, such as:
- Phase IIIA - before NDA submission;
- Phase IIIB - after NDA submission but still before it is approved since it can't be approved before the study is conducted.
G. New Drug Application (NDA) submission: FDA approval
The FDA reviews the results from the clinical trials and other relevant information to ensure that the tested treatment is safe and effective for the patients. If authorized, the new drug/treatment is made available to the general public and becomes a standard of care. It is also tested against additional drugs and treatments. Further studies are conducted if the FDA thinks more information is necessary to determine its efficiency and safety.
In 2015, the FDA approved 96% of NDAs. Interesting, isn't it? This raised a lot of speculation about the FDA and if they are being easy on the companies. But it is important to understand that the agency goes through cycles, approving too much or too less. Other reasons could be that drug companies are getting better at research or choosing areas with higher chances of approval. Whatever the case may be, the FDA is the gold standard for drug and treatment approval.
H. Phase IV clinical trials (post-marketing surveillance studies)
In this phase, the drug approved by the FDA is under continued surveillance to study its long-term effects on a larger population group. This phase is important for answering some important questions regarding the efficiency and safety of the drug or treatment.
What Are the 4 Phases of Clinical Trials?
As highlighted above, there are 4 main phases of clinical trials, i.e., stages of the overall process involving human subjects, and are classified into Phase I clinical trials, Phase II clinical trials, Phase III clinical trials, and Phase IV clinical trials.
Need-to-Knows About Clinical Trials and Their Phases
Before diving into the phases of clinical trials, we want to bring your attention to the following considerations:
- Some aspects of the different phases may overlap or blur. For example, safety information is collected throughout all phases, such as adverse reactions, hypotheses, etc.
- Each phase is a separate study and involves separate design/protocols and enrollment/consenting processes. Patients do not "go through" clinical trial phases, but the drugs/treatments do.
- The different trial phases present different risk-reward ratios for prospective participants. As more information is collected, the trial is considered more likely to be safe and more likely to provide therapeutic benefit to the patient.
- Not all trial phases aim to provide therapeutic benefit to participants, although it is possible for benefits to be experienced during any phase, depending on each person and their specific condition and response to the treatment, as well as study design.
- Clinical trials are of two types; they can be "fixed" (not allowing changes to the protocol while the trial is in progress) or "adaptive" (permitting modification to the study protocol while it is in progress, depending on the results coming in).
- All clinical trial phases are important and necessary for the drug approval process.
- As a participant, you have a basic right to withdraw from the study voluntarily and without needing to provide any excuse or explanation, at any moment, including while the trial is in progress. Your contribution is more valuable if you can commit to the entire clinical trial timeline and stick it out, but you have zero obligation.
- Remember that patient safety is given utmost priority throughout the clinical research process, through all clinical trial phases, and that third-party reviewers are responsible for approving all studies regarding ethics in their dealings with human subjects.
- Participation in clinical research is voluntary, but you are making an extremely valuable contribution to medical science, hopefully improving the lives of people around the world living with your condition, including future generations.
What Is a Phase 0 Clinical Trial?
Phase 0 clinical trials, also known as "exploratory IND studies," were introduced by the FDA in 2006 to learn more about the new drugs without exposing human subjects to the completely harmful and toxic effects. It is designed to streamline the clinical trial process by answering basic questions and saving time and resources.
In Phase 0 studies, the human subjects are given a sub-therapeutic dose of the new drug, not more than a week or so, to study how the drug reacts with the human body, how it is absorbed, and what are the possible side effects, before proceeding to Phase I clinical trials. This helps in eliminating candidate drugs that don't show promising results. Moreover, this phase is not designed to benefit the participants in any way, and the chances of them benefitting from such trials are minimal to null.
Another important thing to note is that Phase 0 clinical trials are not always covered in resources explaining the 4 main phases of clinical trials. They are not a requirement of the drug approval process and are not always conducted because they are different from the other 4 phases.
Phase I Clinical Trials (Dose-Escalation Studies)
Phase I clinical trials aim to study the effects of the new drug or treatment on human subjects. Small groups of volunteers, around 20-100 (typically healthy volunteers, although people with the disease can also participate), are gathered to test if the new drug/treatment is safe, what is the maximum tolerable dosage (MTD) that is unlikely to cause any serious side effects, and what is the best way to administer the drug/treatment.
This phase, also known as the dose-escalation study, does not aim to bring any therapeutic benefit to the participant because little is known about the drug's effect on the human body; thus, safety is ensured. Participants are closely under surveillance and get minimum drug dosage initially; if no side effects are apparent, the dosage is increased. This process continues until the ideal dosage is determined. Simply put, exposure to subtherapeutic doses while maintaining rapid accumulation and safety is the primary goal of the Phase I clinical trial.
Phase I clinical trials last a few months, and approximately 70% of the drugs proceed to Phase II clinical trials.
Phase II Clinical Trials
Also, a part of the dose-finding studies, the Phase II clinical trials involve testing the new drug or treatment over a larger group of volunteers, i.e., 100-300 people with the condition that the drug is designed to manage or treat. This phase aims to find the most successful dose (MSD) where patients receive a therapeutic response without facing serious side effects. It is used to test the safety and efficiency of a drug and gather more thorough information about its side effects on human subjects.
Since the goal is to test the safety and efficiency of the drug or treatment on a larger group of people, it doesn't offer any therapeutic benefit to the participants. However, this phase determines whether the drug/treatment is likely to work for the intended purpose.
Phase II clinical trials can last for a few months up to 2 years, and approximately only 33% of the drugs move on to the next phase.
Phase III Clinical Trials (Pivotal Studies)
Phase III clinical trials involve the full-scale evaluation of the new drug/treatment and are designed to test the safety and efficiency of the new drug or treatment with the standard drug/treatment. This is the most extensive and thorough clinical trial phase and involves the largest number of people, around a hundred to a few thousand, and lasts for around 1 to 4 years, although there is no standard timeline.
Phase III clinical trials can follow different study designs, such as randomized controlled trials (the gold standard), uncontrolled trials, factorial designs, historical controls, and sequential group designs. In RCT, participants are divided into two randomly-assigned groups; control (the group that gets the placebo/standard treatment) and study (the group that gets the new drug/treatment).
Phase III clinical trials are conducted at multiple sites, possibly in different countries, to enroll a wider population and gather larger sample data. Once a drug is found promising, a New Investigation Drug (IND) application is filed to the FDA. The drug is marketed and available to the general population if approved. This is why it is also known as the "pivotal or pre-marketing studies." However, the approval rate is low, and approximately 25-30% of drugs move on to the next phase.
Since this phase focuses on monitoring the side effects and adverse reactions in a long-term wider population setting, it helps gather more safety information. Phase III clinical trials offer the most therapeutic benefits to the participants as the optimal dose has been established, and the study lasts long to collect more accurate information.
Phase IV Clinical Trials (Post-Marketing Surveillance Studies)
As mentioned above, the new drug, treatment, or device is made available to the general population once the FDA approves it. Phase IV clinical trials involve researchers tracking the safety and efficiency of the drug over long-term treatment because although the drug has been tested on thousands of participants, many questions are still left unanswered, such as does the drug manage or eliminate the disease long-term, what are the adverse effects in long-term, etc.
Thus, all studies conducted after FDA approval are included in Phase IV clinical trials and represent a mix between medical research and clinical practice.
Phase IV clinical trials are also termed post-marketing surveillance studies as they study the treatment outcome in real-world situations and analyze the efficiency, long-term effects, healthcare costs, the incidence of adverse effects, and pharmacogenetics (how your body reacts to drugs). These factors can determine if the new drug will be assigned to the general population, removed from the market, or restricted to certain conditions.
The number of participants depends on the number of people using the drug or treatment. At the same time, the length of Phase IV clinical trial is entirely dependent on the information collected and any potential concerns detected.
Conclusion
We hope you feel comfortable with and understand the different parts of clinical research and why they are all important! Participation in clinical research is a brave act that provides immense value to healthcare and people around the world, present, and future, living with various conditions/illnesses. Rest assured that participant safety is given top priority, and remember that it is your fundamental right to choose whether or not to participate and leave the study at any time and for any reason.
Thus, before participating in any clinical studies, ask the following questions and gather as much information as possible to ensure your safety.
- What is being studied, and why do researchers believe this could be an effective alternative?
- What kind of interventions can you be subjected to?
- What are the possible benefits and side effects?
- How long is the study, and will I be paid or reimbursed?
Clinical trials are becoming increasingly "patient-centric" - focused on you, the participant, and making your experience smoother and more enjoyable, so why not head over to Power, where you can find out if there are any trials you are eligible for and may benefit from in only a few minutes.