How to Participate in Mental Health Research

People who volunteer for mental health research and clinical studies are heroes to researchers, clinicians, and patients who benefit from the research findings. Without clinical research participants, it would be next to impossible for scientists and health care providers to come up with new treatments, develop new medications, and understand where to put resources to help scientists innovate so that they can help more people in the future.
Whether your interest in participating in a mental health research study is to gain access to a potentially life-changing clinical trial, better your understanding of your health challenges, be a part of cutting-edge scientific discoveries, or accomplish all three, this article is written with you in mind.
We provide valuable information about what mental health clinical trials are, how they work, who might be eligible to participate, and what you can expect if you decide to apply for a spot in a clinical research study.
- What Are Clinical Trials?
- Who Is Eligible to Participate in Clinical Trials for Mental Health Research?
- Should I Participate in a Clinical Trial?
- How to Participate in a Mental Health Clinical Trial
- What to Expect When Participating in a Clinical Trial
- Protecting Study Participants: Know Your Rights
- Types of Mental Health Conditions
- Breakthroughs in Mental Health Research
What Are Clinical Trials?
There are two basic categories of clinical research: observational studies and clinical trials. With observational studies, researchers stand back and observe people in their normal settings, gathering information and assessing the changes over time. With clinical trials — the type of clinical research addressed in this article — researchers ask participants to undergo some sort of new protocol, procedure, or treatment to gauge its efficacy.
Clinical trials are further categorized into:
- Intervention studies. An intervention study aims to see if a new device, drug, or procedure produces a desired outcome in a person diagnosed with a particular medical condition.
- Prevention studies. Prevention studies gauge whether certain strategies — such as the use of certain medicines, vaccinations, and lifestyle improvements — can help prevent disease.
- Diagnostic studies. Diagnostic or screening studies determine whether a new or modified test to detect the presence of a condition or disease heightens diagnostic abilities.
- Quality of life studies. Sometimes referred to as supportive care studies, these trials test options for improving the lives of people with chronic illnesses.
Clinical trials aren’t conducted in one fell swoop. Rather, they’re broken down into four phases. Here’s how each phase relates to the size and purpose of the overall research study at hand.
- Phase I studies typically involve a small number of participants, usually somewhere between 20 and 80 people. This initial phase determines the safety of the drug or device being studied.
- Phase II studies open up to a larger participant pool, typically 100 to 300 total. While researchers are still determining safety, they now begin to look into the effectiveness of the subject of the research.
- Phase III begins the large-scale trials (1,000 – 3,000 people). Now, researchers are looking to confirm the effectiveness of a drug, device, or procedure being tested. They also look at side effects. If Phase III is successfully concluded, the researchers often seek government approval to make the treatment available to the general public.
- Phase IV research is conducted so that researchers can collect additional information about the treatment’s benefits and risks as they determine the best use of the new medicine, device, or protocol.
In the past several years, clinical trials addressing mental health have increased significantly. In 2007, there were 240 mental health-related clinical trials performed in the United States, a number that rose to 1,611 by 2021.
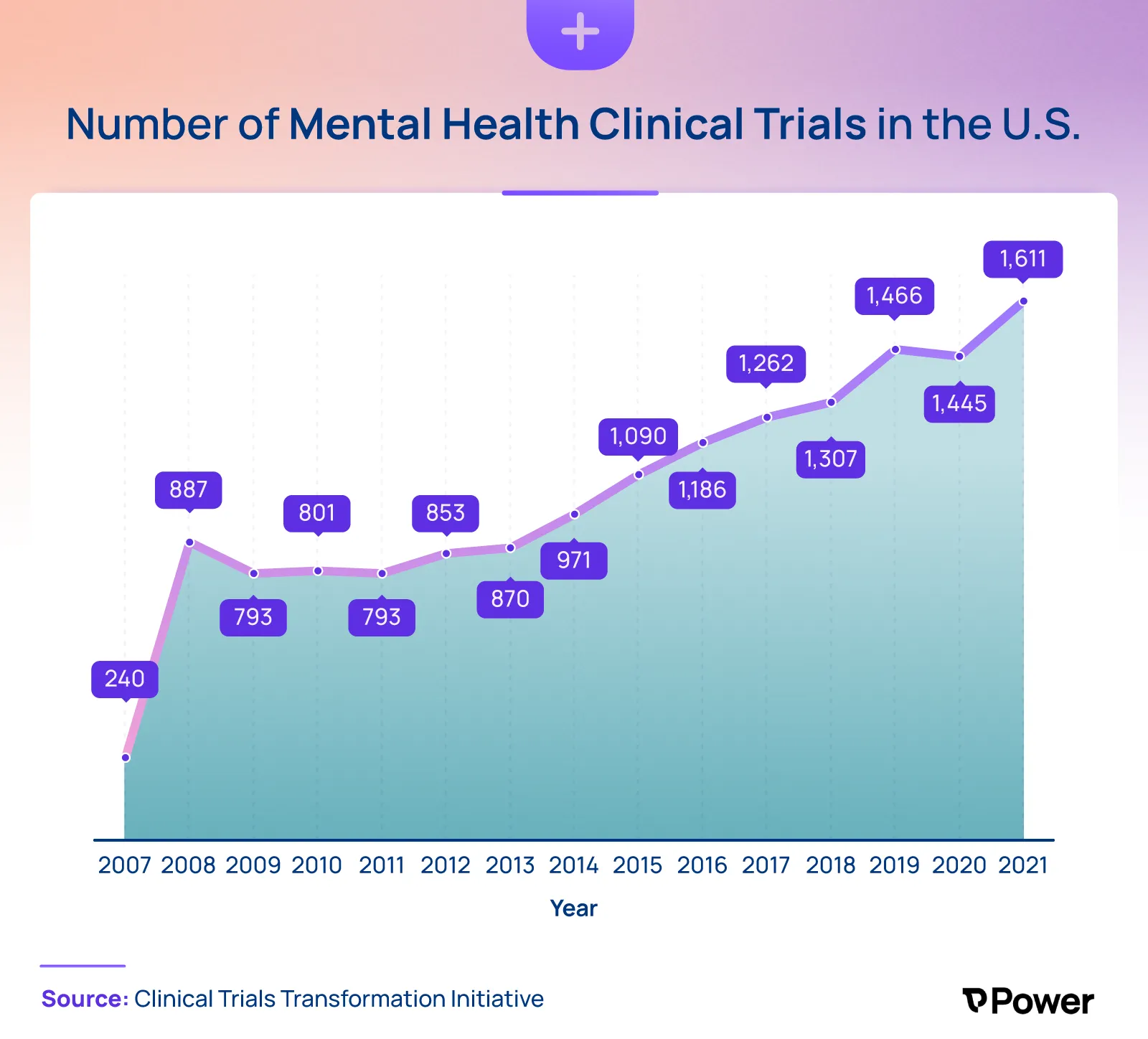
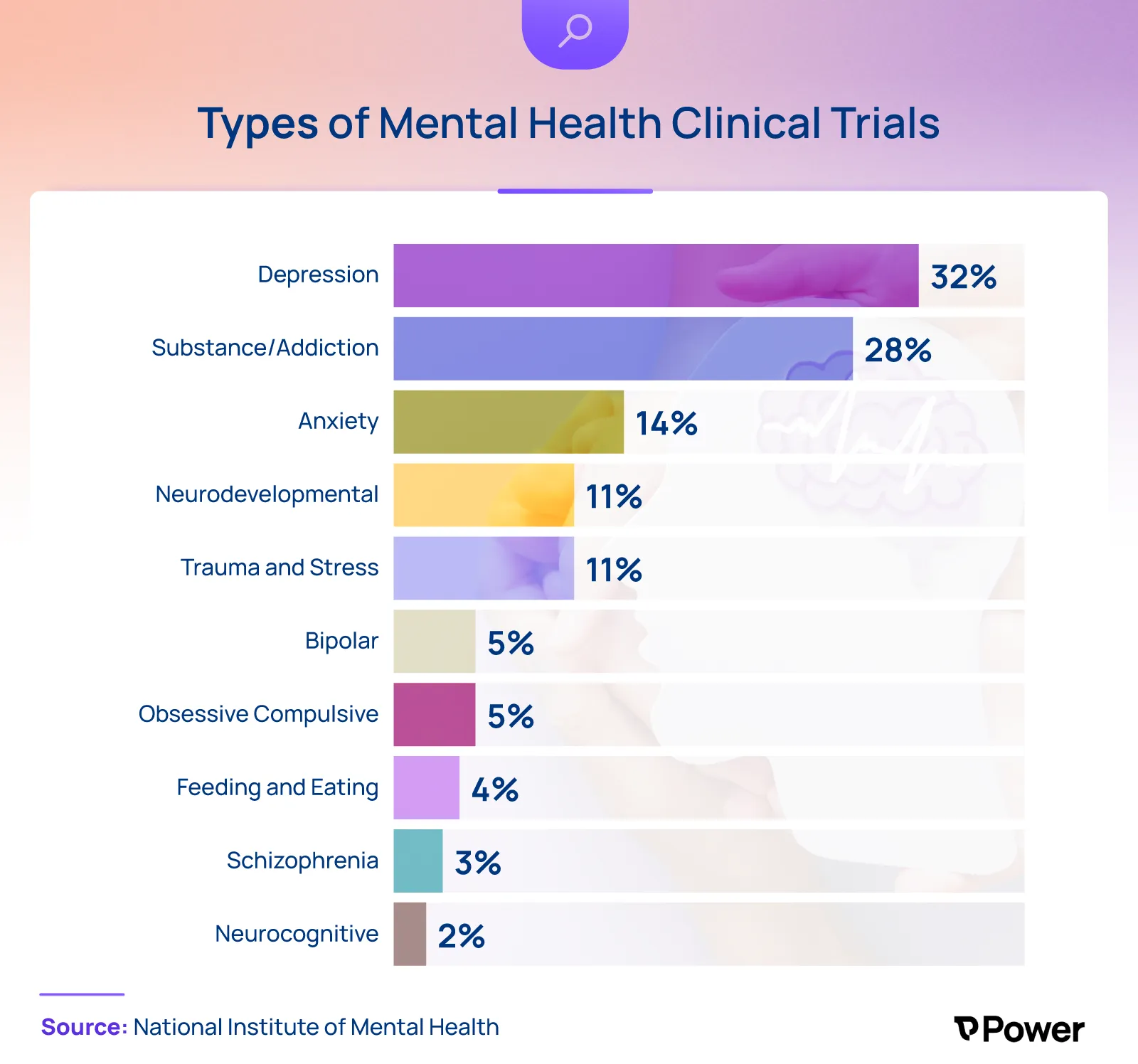
While clinical trials address several different categories of mental illness, more than half seek solutions to two of the most prevalent mental health issues today: depression and substance abuse.
Why Are Clinical Trials Important for Mental Health Research?
Clinical research studies are integral in improving mental health treatments for several reasons. The information gleaned from the studies can advance treatments and expand options. In addition, clinical trials can help guide health professionals in finding ways to identify mental health problems earlier, diagnose them quicker, and treat them more effectively.
The research team typically can gather more informative data with trials on volunteer participants than if they were to perform the research on animals. Important treatment research can progress faster and become available to people who need it with clinical trials.

Should I Participate in a Clinical Trial?
Being part of a research trial can be an immensely fulfilling experience. It can also be a burdensome one. There are legal and ethical protections to help ensure that only well-informed volunteers participate in clinical trials, but that doesn't mean you'll be guaranteed a favorable outcome..
Before you commit to becoming a research participant, you owe it to yourself to carefully examine the pros and cons of volunteering. For instance:
Pros:
- You may develop new insights and learn new information about your condition.
- You could have access to potentially beneficial new treatments before they become available to the general population.
- You’ll be under the care of doctors who are experts in your condition.
- You could receive additional care at no additional cost.
- You’ll be helping researchers obtain knowledge that could be life-changing for others.
Cons:
- You might be disappointed at the results you receive in the trial.
- You could experience unpleasant or even serious side effects from the new drug or treatment.
- You may have to devote significant time and energy to meet the requirements to participate in the study.
- You may never know the results of the research or benefit from it.
- You could end up being removed from the study, as there are no guarantees that you’ll remain a viable candidate for participation.
Who Is Eligible to Participate in Clinical Trials for Mental Health Research?
Whether you’re eligible to participate in a clinical trial for mental health research depends on several factors specific to the study. While each research institution develops its own set of criteria, it’s typical for participants to be screened for:
- Past and current illnesses
- Medical history and relevant diagnoses
- Past treatments and outcomes
- Current treatments and outcomes
- Demographic criteria, such as age and sex
Once an institution determines that you qualify for the trial, you’ll have an opportunity to gather all of the information needed to give informed consent to participate in the research study. You must understand all of the potential risks and benefits before signing on to be a study subject.
How to Participate in a Mental Health Clinical Trial
If you decide to pursue participation in a mental health clinical trial, talk to your family members and/or caregivers about the support you’ll need from them. Then, take these actions to get started.
1. Find Available Trials
You can find mental health clinical trials in several places. For example, you can:
- Look for NIMH studies particular to your mental disorder. You can find a list of adult mental health research studies by disorder on the National Institute of Mental Health (NIMH) website. You can find information on current studies related to depression, anxiety disorders, and schizophrenia, to name a few. You can also find information on being a healthy volunteer in research on topics like how the brain works. The NIMH also has opportunities for child participants in clinical trials and studies geared toward children and adolescents.
- Ask your physician to refer you to a study. Your health care provider may have information on local studies. They should be able to direct you to research groups in your community or even national research studies that would be appropriate for you.
- Consult a reputable online service. Online platforms like Power provide an easy way to browse clinical trials by condition, location, and drug type, as well as connect with experts and researchers to guide you through the process of choosing and signing up for a trial.
2. Determine If You Qualify
Most researchers have exact criteria for the kind of individuals they need for their clinical trials. When researching if you’re likely to qualify for a trial, explore the following:
- Whether you meet the threshold criteria for participation. If you’re seeking to enter a trial as a patient volunteer, you may have to show that you’ve received a certain diagnosis, are at the stage of the disease being studied, and that any treatment you’ve already undergone is compatible with the study parameters.
- If your comorbidities disqualify you. Sometimes, even if you meet the threshold requirements for the trial, you might be deemed ineligible if you have other health issues. For example, if you’re seeking to participate in a clinical trial for a new antidepressant medication, you might be disqualified if you’re being treated for a seemingly unrelated health issue such as diabetes.
- Whether you meet the particular demographic requirements for participation in the study. This could include factors like age, gender, race, or geographic location.
- Whether you have the necessary support system in place. Often, before you’re accepted into a clinical trial, you’ll have to demonstrate that you have the resources necessary to ensure you’ll be able to comply with study requirements. This could include anything from having reliable transportation to appointments, a history of compliance with medication protocols, and/or a care partner to assist with reporting.
A platform like Power can help you determine your eligibility for a particular study. For example, if you’re interested in participating in a clinical trial of the drug AVP-786 that’s designed to treat negative symptoms of schizophrenia, you’ll want to review the trial’s eligibility criteria and find that to be accepted into this study, you must:
- Be between 18 and 65 years old
- Have a DSM-V diagnosis of schizophrenia confirmed by the Mini International Neuropsychiatric Interview (M.I.N.I) Version 7.0.2
- Be receiving and be adhering to your dosing schedule for a second-generation atypical antipsychotic drug (SGA)
- Have well-controlled positive symptoms and prominent negative symptoms as defined by Positive and Negative Syndrome Scale (PANSS) criteria
- Have a reliable informant (e.g., case manager, social worker, family member) who can spend an adequate amount of time with the participant to be able to address behaviors, activities, and symptoms
- Be available to participate in the trial over a 15-week period at one of the 53 designated trial locations in Florida
The platform’s pre-populated questionnaire will take you through each criterion and, based on your answers, help you determine your eligibility for the program.
3. Reach Out to the Study Organizers
Once you’ve found a clinical trial you’d like to join and have determined that you likely qualify, you’ll want to contact the study organizers to explore the next steps in applying. Some study organizers — such as researchers running NIMH studies — provide their contact information in the description of the study. You can email the contact or call them at the number provided to schedule an initial screening by phone or online.
If you’re applying for a study through an online platform like Power, you’ll be pre-screened directly on the app and then invited to leave your information with the relevant researcher, who will contact you by phone.
4. Follow Up With Any Questions About the Study
Understand what you’re signing up for by closely reviewing the study description. You’ll receive an informed consent statement that details the study with information about the length of the study, what will be required of you, and what will go on in the study.
Remember that there can be rewards for participating in a clinical trial, but there are also risks. Before committing to a research study, make sure you can fulfill the obligations of the study and that you can tolerate any of the negative impacts participation may have.
Make sure you can answer the following:
- How will the study help treat my disorder?
- How will my participation affect my life (take up time, cause side effects, etc.)?
- Are there any serious health risks that could occur if I participate?
- Will I be inpatient or outpatient?
- Will I be paid for participating in the study?
- How long will the study last?
5. Participate (If Selected)
If you’re chosen to participate in a mental health clinical trial, only commit if you can answer yes to the following questions:
- Can you attend all the appointments required?
- Will you be able to follow the process protocol?
- Can you provide the updates that the clinicians need on time?
- Do you have support resources if you experience side effects?
What to Expect When Participating in a Clinical Trial
While every clinical trial has its own rules, parameters, and protocols, you must be mentally and physically ready for the experience, are prepared to weather any storms that come up during the study, and have ample support and resources in place before, during, and after the trial.
While all of the following might not apply to the trial you’re participating in, the chances are great that you’ll be able to relate to one or more of the experiences outlined below.
- You may have to undergo a physical exam. While some studies will accept you based on your medical records and physician’s referral alone, others require medical examinations before you can participate. Depending on the study, this could involve measurement of your height, weight, temperature, and blood pressure, as well as undergoing a heart trace (electrocardiogram), blood and urine analyses, and any other diagnostic tests required by the study protocol.
- You may spend time in the hospital. Depending on the type of clinical trial, you may have to be in a controlled setting like a hospital or clinic for all or part of the study.
- You may have to submit to testing on a strict pre-determined schedule. It’s not unusual to make frequent trips to the study center or lab for tests.
- You might be required to keep a detailed health journal. Some studies require volunteers to record certain information about their health, reaction to the treatment, diet, and other details of their day-to-day lives. Even if this isn’t a requirement of your study, it’s a good idea to keep a journal so that you remember issues to discuss with the research team or your physician.
- You may find varying degrees of support from friends and family members. While you can hope that your loved ones will support your participation in the trial, you might be surprised by some reactions. Think about who to inform about what you’re going through and how to ask for any support you’ll need.
- You may have to carefully plan for work absences. Some clinical trials might require you to be away from home and work for a period of time. With others, you might find that you can’t continue your normal routine during all or part of the trial, including being at work. You may need to pre-arrange for time off if needed.
- You may have to pay for travel and other expenses. Some trials cover travel expenses, and some don’t. Make sure you know how you’re going to arrange for and pay for any travel or ancillary expenses that could come up during the trial.
- You may become sick. Whether from a reaction to the trial meds or just because life happens, make sure you have a support network in place to render aid if you become sick during the trial.
Protecting Study Participants: Know your Rights
Clinical trials in the U.S. are subject to a system of scientific oversight designed to make sure study participants remain safe. The role of scientific oversight is to monitor studies as they progress and stop trials if the strategies or treatments are proving harmful. Scientific oversight can also result in a trial being stopped early in cases where the benefits of the new treatments or protocols are established early.
Scientific oversight is accomplished through one or more of the following:
- Institutional review boards (IRBs). All U.S. clinical trials are required to have an IRB. IRBs are independent committees created by the institution sponsoring a study. Comprised of physicians, statisticians, and members of the community, IRBs ensure that clinical trials are conducted in an ethical manner and for ethical purposes and that the rights of all participants are protected.
- Office for Human Research Protections (OHRP). The OHRP is charged with protecting the rights and well-being of all participants involved with research trials conducted or supported by the U.S. Department of Health and Human Services (HHS). The OHRP provides oversight of the institution’s IRB.
- Data Safety Monitoring Board (BMB). A DSMB reviews data from a clinical trial to identify any safety problems or other patient risks. All National Institutes of Health (NIH) phase II clinical trials, large trials that compare alternative strategies for diagnosis or treatment, and certain early-phase trials that involve high-risk procedures or vulnerable patients (e.g., gene therapy or child subjects) must undergo DSMB reviews during the trials. The DSMB is staffed by research and study topic experts.
- Food and Drug Administration (FDA). The FDA oversees all U.S. clinical trials testing new medicines or medical devices. Before testing on humans, the researchers must submit an application to the FDA. The FDA provides oversight and guidance during the studies.
What Is Informed Consent?
Before agreeing to participate in a clinical trial, you must undergo the informed consent process. The researchers are required to give you all of the facts surrounding the trial before you sign up to take part in the study.
After explaining the treatments, tests, and protocols you’ll undergo during the trial — as well as all of the potential risks and benefits involved — you’ll be asked to sign an informed consent form that reiterates all of the information provided. You can’t start the trial until you sign the form.
Informed consent continues throughout the trial. If a matter comes up that hasn’t been previously disclosed, the researchers are required to inform you of it while the research is underway. You can revoke your consent at any time.
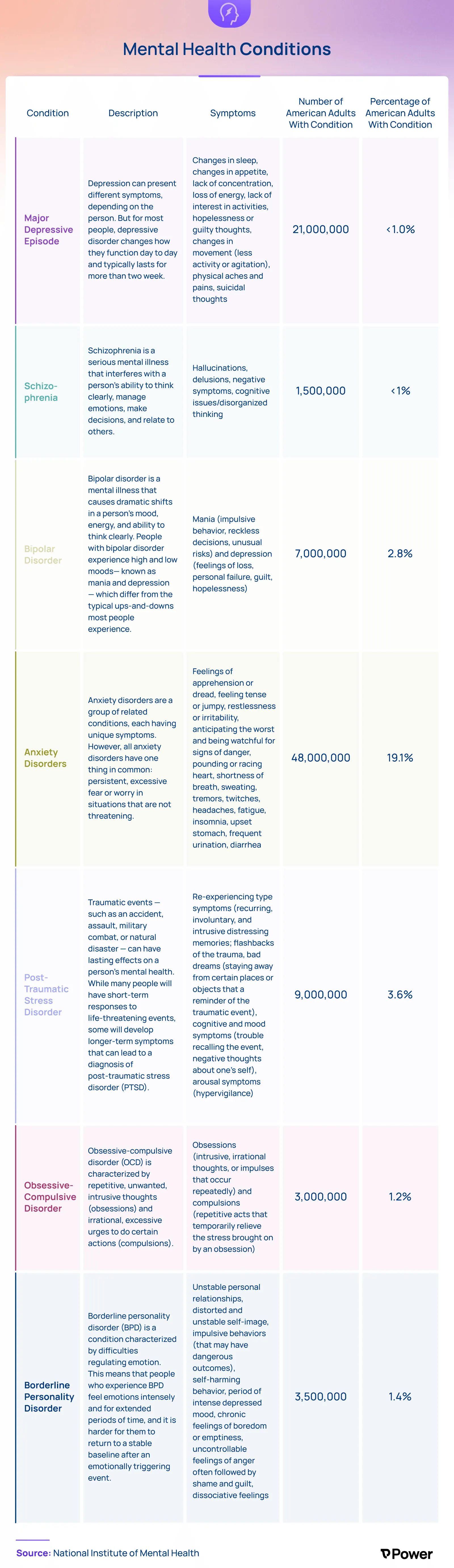
Types of Mental Health Conditions
Clinical trials can improve the impact of mental health services for a wide variety of patients. In fact, half of all lifetime mental illnesses begin by 14 years old, and 75% begin by age 24.
These are a few of the many conditions that may need volunteer participants.
Major Depressive Episode
8.4% of American adults have a major depressive episode condition, which is a mood disorder. Major depressive episodes can have several different symptoms, such as changes in appetite and sleep habits, lack of attention and interest in activities, hopelessness, guilt, and sometimes suicidal thoughts. This condition typically lasts over two weeks.
Schizophrenia
Schizophrenia is a psychotic disorder, and it’s much less common than a major depressive episode but still affects 1.5 million American adults. It’s a serious mental health disorder that inhibits a person’s ability to think clearly and relate to others. Common symptoms include hallucinations, delusions, and disorganized thinking.
Bipolar Disorder
There are 7 million or 2.8% of American adults who have bipolar disorder. The illness causes dramatic shifts in a person’s mood and energy. The person will have highs (mania) and lows (depression) that can affect their ability to think clearly. While medication can help, this mental illness isn’t curable.
Bipolar disorder is often inherited — 80% to 90% of individuals with bipolar disorder have a relative with it.
Anxiety Disorder
Anxiety disorders are a group of related conditions. A staggering 48 million people, or 19% of American adults, have an anxiety disorder.
They all present a large amount of fear and worry in the person. Feeling apprehensive, worried, jumpy, and fearing the worst is common with an anxiety disorder. The person may also have symptoms like a pounding heart, shortness of breath, fatigue, and an upset stomach.
Post-Traumatic Stress Disorder
Post-traumatic stress disorder (PTSD) results from a previous injury or stressful event. Nearly 4% of American adults have this condition. Re-living the trauma of the event is one symptom of PTSD, as are bad dreams, trouble recalling the event, and avoidance of locations or activities that remind them of the event. Assessments can be made on whether the person needs medication, therapy, or both for treatment.
Obsessive Compulsive Disorder
As many as 3 million American adults have obsessive-compulsive disorder (OCD). People with OCD experience a pattern of unwanted thoughts and fears (obsessions) that lead them to do repetitive behaviors (compulsions). These obsessions and compulsions interfere with daily activities and cause significant distress. While OCD usually includes both obsessions and compulsions, it’s also possible to have only obsessive symptoms or only compulsion symptoms.
Borderline Personality Disorder
Borderline personality disorder (BPO) can result in someone feeling emotions more intensely than others, making it difficult for them to reach a stable level of emotion after a highly charged experience. People with BPO may have unstable relationships, a distorted self-image, and uncontrollable anger. About 3.5 million American adults, which is 1.4% of the population, have BPO.
Breakthroughs in Mental Health Research
Mental health research produces promising breakthroughs every year. As reported by the Brain & Behavior Research Foundation — the largest private funder of mental health research grants — the following is just three of the many recent breakthroughs that have occurred from mental health research.
- The results of a clinical trial suggest that repeated infusions of the drug ketamine over a two-week period can significantly reduce chronic PTSD symptom severity in many patients while also helping to reduce depression symptoms that often accompany PTSD.
- In the area of depression treatment, researchers reported initial success in using a novel, highly personalized, and technologically advanced deep-brain stimulation (DBS) approach. The treatment was successful in rapidly relieving symptoms, eliminating suicidal thoughts, and by four months, achieving remission in a patient with lifelong and severe treatment-resistant depression.
- In a double-blind, placebo-controlled trial involving 28 patients diagnosed with schizotypal personality disorder (SPD), adding the drug guanfacine to a proven therapy program to treat cognitive deficits led to significantly greater improvement in the reasoning, problem-solving, and functional skills, as well as social cognition in many of the guanfacine-treated participants.
The Popularity of Teletherapy
Telehealth is changing the mental health care landscape. As more patients turn to online therapy as an option for treating mental disorders, researchers are beginning to study the efficacy of telehealth treatments.
For example, a recent randomized clinical trial revealed that a one-day interactive workshop based on cognitive behavioral therapy (CBT) and delivered via Zoom reduced symptoms of women experiencing postpartum depression.
Faster-Acting Antidepressants
A significant breakthrough for those with depression came with the discovery that acute ketamine infusions can produce rapid and sustained antidepressant effects, revolutionizing the way mental health professionals understand both the pathophysiology and potential treatments for depression. This type of research has the potential to significantly and positively impact the lives of the 280 million people who have depression worldwide.
Better Insurance Coverage for Mental Health Issues
Research on how access to mental health services based on insurance coverage impacts outcomes has brought some important insights to light. For instance, a recent study of how mental health care varies by demographics and health insurance coverage revealed that a significant number of uninsured adults reporting moderate to severe symptoms of anxiety and/or depression didn’t receive the care they needed.
As researchers continue to explore the relationship between insurance coverage and mental health care access, it’s hoped that barriers to treatment will decrease as innovative ways to reach underserved populations increase.
Greater Acceptance of Cognitive Behavioral Therapy
Mental health research has been invaluable in gauging the efficacy and acceptance of certain types of treatments and therapies. For example, the increased acceptance of CBT has resulted from numerous studies demonstrating that CBT is often as or more effective than other types of therapy or even psychiatric medication.

Find a Clinical Trial Near You With Power
If you’re ready to find a clinical trial but aren’t sure how to start your search, Power can help. Power is a patient-friendly platform where people like you can easily research, access, and sign on to appropriate clinical trials. Our one-stop solution makes it easy to search through all the clinical trials available in the U.S. by condition, location, and participant criteria.
Learn more about how Power can empower you to join a clinical trial that’s right for you.