Clinical Data Management Process Flow Chart
What is clinical data management (What is CDM)?
Clinical data management (CDM) refers to the processes of collecting, cleaning, and managing data obtained during clinical trials, and also involves an extensive planning stage in order to set up databases and CRFs according to the trial’s protocol and specific data requirements. Through implementing standardized procedures for data collection, storage, and analysis, good CDM practices ensure that clinical data is complete, accurate, and reliable, and that study results are valid. In other words, CDM is essential for guaranteeing data integrity in clinical trials, with additional impacts in trial quality, research integrity, patient safety, and regulatory compliance.
Typically, a clinical data manager is responsible for overseeing these processes, starting with the development of a data management plan (DMP), setting up and overseeing the implementation of clinical trial data management systems (CDMS), and monitoring data handling and enforcing data management protocols, throughout the duration of the trial and across all sites. By ensuring accurate capture of information from study participants via selected data collection methods into well-designed, study-specific case report forms (CRFs), the data management team makes sure that the right data is collected in the right way to support analysts to draw scientifically robust conclusions about the intervention under study. Additionally, strong documentation practices facilitate trust and transparency, regulatory compliance and audit trails, and reproducibility of the study.
In this article, we summarize and present the clinical data management workflow in the form of a clinical data management process flow chart. For a deeper dive into clinical data management and its individual steps, see our in-depth article on data management in clinical trials.
What are the 4 phases of clinical data management?
The clinical data management process is sometimes broadly categorized into four phases: data collection, data cleaning, data analysis, and reporting. This works as a general overview, but in reality each of these phases involves numerous individual steps that are each deserving of considerable attention in a well-developed data management strategy.
What are the steps in CDM? The clinical data management workflow
We prefer to use a 10-step clinical data management workflow we have adapted from other resources. Here, we present a brief overview of each step and what it consists of, before presenting this information in graphic format as the clinical data management process flow chart.
0. Protocol development
While not technically a data management step, having a finalized and well-designed study protocol is a prerequisite. The protocol will outline specific objectives, inclusion/exclusion criteria, health outcomes to be assessed, methods for collecting data relevant to these study endpoints, etc. At this point, it should likely be clear which, if any, electronic devices and systems are to be used as part of the trial – for example electronic data capture (EDC) tools, wearable devices, a clinical data management system (CDMS), an electronic quality management system (eQMS), etc. Clinical data managers or a research data coordinator will normally be at least somewhat involved in protocol development since the data process should be incorporated fundamentally within the research methodology.
1. Database design
A structured database will need to be created that reflects the particular study design, enabling data to be captured in an organized manner, considering proper formatting, automated edit checks, integration with any other software tools, etc.
2. CRF creation and medical coding
Next, a customized case report form (CRF) should be created. The CRF is the record and medium for capturing study data for each patient, and can be designed for use in paper or electronic (eCRF) format (or both). Paper-based CRFs will need to be transcribed into electronic study records (manually or through OCR scanning technology), whereas eCRFs can be integrated directly into the CDMS or other software tools.
3. Creation of the data management plan (DMP)
Once the database and CRF design are finalized and it is clear which software solutions are to be used in the study in general and for data processing, the data manager can set forth a finalized data management plan. The DMP should be a comprehensive document accounting for all factors that will or might influence study data and/or its handling in any way, and will likely include standard operating procedures (SOPs) for various aspects of data handling and monitoring
4. Quality assurance and user acceptance testing (UAT)
Particularly when there are EDC tools, ePRO, or wearable devices involved in the study, it is of vital importance to validate these tools and perform UAT to ensure the patient experience is manageable and uniform. However, electronic systems also need to be validated at study sites, and investigators and other study staff are also considered end-users of these tools. Depending on who is using which tools and for what purpose, QA and UAT can involve a variety of steps. Any issues that might compromise trial data quality or impede smooth operations should be addressed before the trial begins.
5. Data collection
At this point, the study can begin. Sites and investigators will begin collecting patient-related information in case report forms (CRFs), beginning during the informed consent process and continuing through to the end of the study (and perhaps even continuing after, depending on the follow-up requirements).
6. Data validation and cleaning (discrepancy management)
While aspects of data validation such as edit checks are likely performed as a routine part of data collection, especially when electronic tools/devices are involved, there still needs to be a formal audit to ensure that the study dataset is thoroughly validated and cleaned. This includes query management and resolving any discrepancies identified during validation. Each database entry should be validated in accordance with its importance to the study integrity and quality; in other words, many believe that it is overkill to verify every single data point, and instead suggest prioritizing validation of the data that could have real effects on the study results or patient safety.
7. Database lock
After careful review and resolution of all queries and discrepancies, the cleaned and validated dataset or database is “locked,” meaning no further changes can be made to the dataset (although the intermediate step, “soft-lock” typically allows for changes to still be made). The point of database locking is to ensure the integrity of trial data, and thus the validity of the statistical analysis and the conclusions drawn therefrom. See our article on database lock in clinical trials for a more-detailed look at data lock and database soft lock and hard lock states. If it wasn’t done as part of the final validation and cleaning step, this step should include data de-identification to protect subject privacy and confidentiality.
8. Statistical analysis
At this point, the locked database is sent to statisticians for analysis of the study data. Blinded datasets may require coding or other treatment to maintain the blind through analysis (in triple-blinded studies).
9. Reporting
Reporting involves the formal communication of the results of the data analysis to study sponsors and other stakeholders, in such a way that the study comes full-circle and the hypotheses and research questions originally set forth can (hopefully) be answered.
10. Archiving and close-out
Data management for clinical research continues through to the end of the study, after which point there are still requirements related to archiving data (storing it for predetermined periods of time for regulatory purposes) and handling the data during close-out activities.
In the next section, we present this data management workflow in a visual flow chart, which we hope can serve as a starting point or template for customizing your own flow chart adjusted to the specific trial designs you’re working on.
Clinical data management process flow chart
A flow chart can support efficiency in clinical data management by providing a visual representation of the entire data management workflow. It can serve to help sponsors, data managers, investigators, and stakeholders quickly reference data handling steps, facilitating a clear understanding of the entire data management process and its dependencies, minimizing errors or delays.
The flow chart provided here can be used a base and then tailored to match the unique DMP and workflow of the trial at hand, and can be annotated to include details such as the specific tools or software used for different steps, quality control measures, data sharing or reporting requirements and time points, and who is responsible for or involved in each stage.
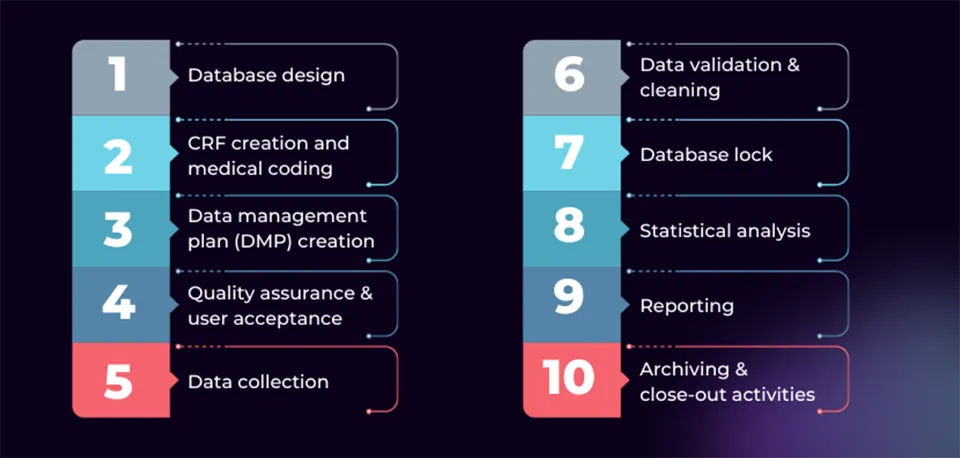
Furthermore, process flow charts can enable identification of potential bottlenecks or areas for improvement within the data management workflow. This can promote continuous quality enhancement by facilitating discussions and supporting stakeholders in implementing changes that enable increased accuracy and efficiency in clinical trial data management and in trial operations overall.
Conclusion
In conclusion, a unique and detailed process flow chart can be a good support for effective clinical data management. Good CDM practices ensure data consistency, accuracy, quality, patient safety, transparency, regulatory compliance, and adherence to data management protocols and reporting requirements. As data management transverses almost every aspect of clinical trial operations, and CDM tools and CDM software systems are increasingly leveraged by sponsors and data managers, the complexity of the data management landscape lends itself to visual aids that facilitate logistics, organization, and monitoring. That’s precisely where the clinical data management process flow chart comes in.
Additional references for further information on clinical data management workflows
Outline of Clinical Data Management | IJSHR
Data management in clinical research: An overview - PMC
General flowchart for clinical research data management | Download Scientific Diagram
Flow chart showing the process steps in data management | Download Scientific Diagram