What is a Contract Research Organization? Plus, Top 5 CROs to Check Out in 2022

What is a CRO?
A contract research organization – CRO – is a company that provides outsourced services related to drug development and clinical research. Pharma and biotech firms and other sponsors may rely on a CRO for support in one or more aspects of developing and bringing new drugs and medical devices to market.
Keep reading as we explore the role of CROs in drug development, what services they offer, and why many sponsors work with one. We’ve also included a list of the top 5 CROs, and questions to ask before signing a contract with a CRO.
What Do CROs Do?

The main role of CROs is to carry out activities that the sponsoring pharmaceutical company cannot or chooses not to do in-house.
Pharmaceutical companies often count on both internal and external resources, and sometimes they need assistance with tasks that fall outside of their core capabilities. Most modern CROs provide services spanning the drug development pipeline, from running entire clinical trials to individual services such as data management, patient recruitment, protocol development, drug manufacturing, and the list goes on. CROs themselves might partner with other companies to provide robust services across the full spectrum of clinical trial activities. For example, CROs might partner with Power as a part of a multi-layered recruitment strategy for a trial.
The goal of a CRO is to provide streamlined services to pharmaceutical companies in order to help them get their new drugs developed, tested, and approved. It’s often more resource efficient and quicker to outsource certain aspects of clinical development, allowing sponsors to focus on their core operations.
For example, let’s say a biotech company has developed a new drug that looks promising in preclinical laboratory tests, but they’ve never conducted a clinical trial. It would generally be much more straightforward and efficient to partner with a CRO to conduct clinical trials than to dive into the complex regulatory landscape and risk inefficiencies or failures in the clinical testing of their promising new drug. Although the biotech firm would still be the sponsor of the trial, retaining responsibility for regulatory compliance, the CRO would be responsible for recruiting patients, administering the study drug, monitoring patients and overseeing the trial, and reporting the results back to the company. Each of these aspects is, in and of itself, a whole world that requires specialized expertise. It makes much more sense to outsource these aspects to dedicated professionals.
See the image below for some example services offered by CROs:
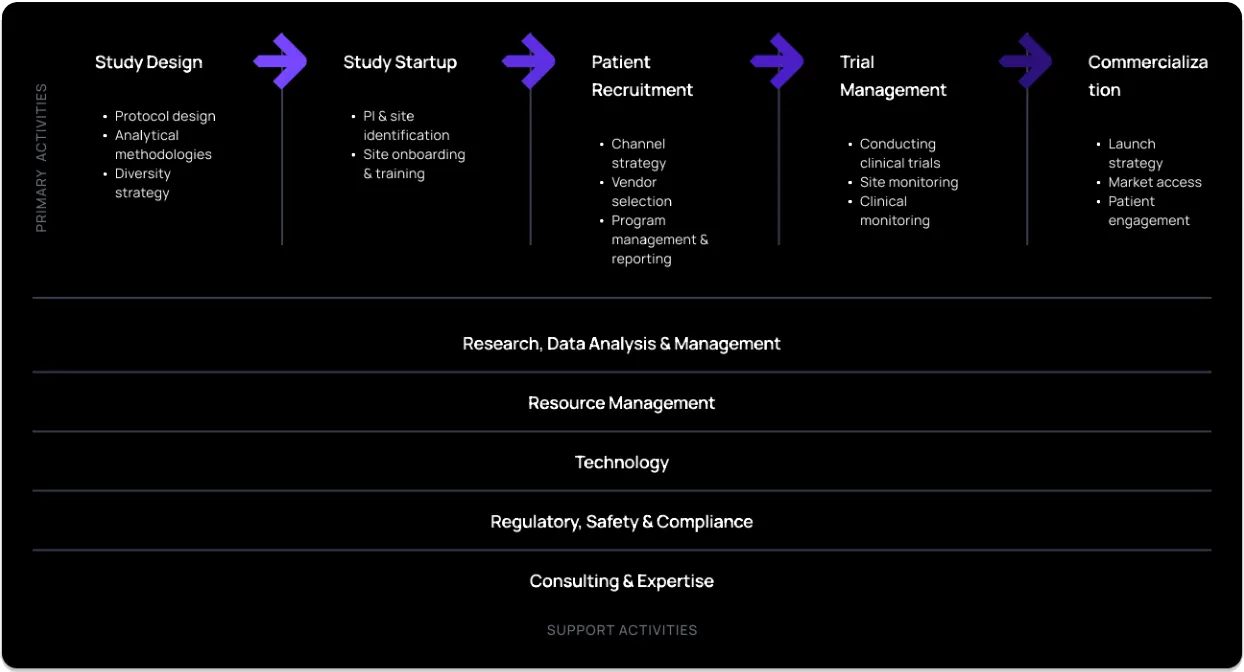
Are contract research organizations the same as clinical research organizations?
Contract research organizations (CROs) may get involved in all aspects of the clinical development process, from initial drug discovery through to pre-clinical and clinical trials and post-marketing surveillance studies. A CROs might help design a study, recruit patients for the study, perform laboratory tests, monitor patients during the study, analyze study data, prepare study reports for the sponsor, and present reports or summaries to regulatory bodies.
Clinical research organization is a less-common term which might be used to refer to a specific type of CRO that specializes in conducting clinical trials for pharmaceutical companies and other sponsor organizations. Thus, where a CRO might offer comprehensive service across the clinical development spectrum, a clinical research organization would be focused primarily on conducting phase I through phase IV studies for new drugs and medical devices.
Why do sponsors work with CROs?
CROs play an important role in pharmaceutical and biotechnology product development. Here are just some of the reasons why sponsors may decide to work with CROs:
1. Specialized operations and cost control
Building and managing clinical trial operations completely in-house is complicated, expensive, and requires specialization in a multitude of areas. The rise of CROs meant that pharmaceutical companies no longer needed to own all of their own scientific and clinical research facilities. Full-service CROs offer sponsors a complete set of solutions, allowing them to delegate as much or as little of the overall clinical development operation as they desire so as to optimize resource use and development timelines.
2. Expanded access to technology
CROs offer various technological solutions to support sponsors in designing, conducting, and managing clinical trials. Many of these tools are specific to clinical trials, and would not be commonplace in a lab-focused pharmaceutical company, for example. Sponsors can choose to leverage these technologies or product suites depending on the needs of each unique study. Some examples of eClinical technologies that sponsors may use include: planning tools (protocol design, patient enrollment, etc.); site management tools (activation, payments, etc.); modern patient recruitment tools (e.g., Power); clinical trial management systems (CTMS); clinical data management systems (CDMS); analytics platforms.
3. Ability to handle large amounts of data
There are a lot of moving pieces involved in successfully running a clinical trial. CROs with experience in clinical trials will have efficient data handling procedures in place, making them a reliable partner for this complex aspect of modern trials which often draw from various data sources such as wearable devices, EHRs, eCOA/ePRO interfaces, etc.
4. Streamlined regulatory affairs (FDA regulatory compliance)
CROs typically have experience working with regulatory agencies such as the Food and Drug Administration (FDA). The FDA regulates the entirety of clinical trials, ensuring that ethical research guidelines and patient privacy safety standards are upheld. Sponsors must adhere to a multitude of FDA and HHS guidelines when conducting any clinical trial. Regulatory affairs is a complex landscape requiring familiarity with various parallel and overlapping regulations, as we discuss in our explainer on regulatory compliance in clinical trials. An experienced CRO will be able to help pharmaceutical companies get their products to market faster by facilitating regulatory submissions and navigating the intricacies of guidelines laid out in Title 21 and 45 CFR, GCP, HIPAA, etc. Partnering with a CRO offers an attractive alternative to hiring and training internal experts specializing in regulatory affairs, particularly for sponsors who aren’t conducting trials frequently.
5. Multi-disciplinary expertise leads to quicker and more effective trials
CROs often consist of a team of professionals with diverse backgrounds and specializations, which means that they can offer the appropriate expertise needed to effectively manage various aspects of clinical trial operations.
What to ask when choosing a CRO partner for clinical research

With the massive offer of CROs currently available, it’s important to realize the weight of the decision when selecting partners for clinical research. As the sponsor, regulatory compliance falls entirely under your responsibility, so choosing a trustworthy partner with a proven record of compliance can help you be sure your trial is in the right hands. Partnering with an incompetent or inexperienced CRO may have the opposite effect of streamlining trial timelines and resource usage, causing delays and risking valuable resources or even patient health.
The following 3 points act as a guideline for choosing a potential CRO before signing a provider agreement with them for a clinical trial, regardless of the extent of their involvement.
1. Obtain trustworthy information. It's important to gather as much information as possible about a potential research partner, as there is significant weight placed on such agreements. The website of the CRO itself will not tell the whole story; it is a good idea to look for real reviews and feedback from other companies who have worked with the CRO.
Make sure that the CRO you are considering has an established track record of success with past and current clients. Check that the CRO has an active online presence and transparent contact information, along with active client support.
The CRO should also have a good reputation among its employees; complaints by employees might be a red flag, particularly if they’re related to protocol deviations, non-compliance, mismanaged client disputes, internal uncleanliness or disorganization, or other issues that could impact patient safety and regulatory compliance.
2. Check that the CRO is up-to-date with regulatory standards and accreditations. The regulatory landscape is updated frequently, so it’s important to verify that the CRO in question has been active recently and has up-to-date authorization and accreditation for the specific operations you are considering contracting them for. If you can’t find this information on the website, contact them and have an open discussion to get clear answers.
3. Verify that the CRO has real expertise in the specific task you wish to outsource. This may seem obvious, but just because a given CRO is world-renowned in conducting clinical trials does not mean they will be experts in analyzing your specific data, particularly if you’re working in rare diseases or other niche areas. Direct experience in a similar therapeutic field and trial type is important, as it translates into efficiency and tells you that they will be comfortable navigating the particular waters you’re entering together. After all, the point is for the partnership to make your operations smoother, and for that, you need a partner with relevant experience.
What Are The Top CROs in 2022?
The contract research organization (CRO) industry is rapidly growing, and it's easy to see why due to the unique roles they fulfill in pharmaceutical and biomedical research.
We’ve curated a list of the top 5 contract research organizations (CROs) in 2022:
1) IQVIA
IQVIA is a leading healthcare technology company providing integrated services spanning the entire healthcare and clinical development spectrum. IQVIA offers solutions for clinical trials and research/development, real-world evidence studies, safety and regulatory compliance, and commercialization, and features a vast suite of technological tools powering “smarter healthcare.”
Labcorp Drug Development, formerly Covance, is a contract research organization that offers drug development, clinical trial, and laboratory services to the pharmaceutical industry. The company's mission is to help advance patient care by providing clients with high-quality services and solutions.
Labcorp was founded in 1996 and has since grown into one of the world's largest CROs, with over 40 offices worldwide.
3) PPD
PPD is part of Thermo Fisher Scientific, serving as the clinical research services arm of the research giant. PPD is known internationally, having conducted clinical trials in over 100 countries, and currently consisting of over 35,000 employees worldwide.
PPD offers a comprehensive range of clinical development and laboratory services, providing sponsors with customizable solutions backed with a proven track record of professionalism and success.
4) ICON
ICON plc offers a full range of consulting, clinical development, and commercialization services to pharma, biotech, and other healthcare sector actors. Founded in Dublin, Ireland in 1990 and now counting on offices in 46 countries worldwide, ICON has a global team of experts and extensive experience in a wide range of therapeutic areas.
5) Medpace
Medpace provides full-service clinical development services aimed particularly at biotech firms, with services ranging from study start-up through to monitoring, pharmacovigilance, regulatory affairs, and even medical writing. Founded in 1992, Medpace now operates in 6 continents and their teams of medical, operational, and regulatory specialists support their “therapeutically-aligned” scientific expertise and the delivery of their full-service, solution-oriented trial execution model.
Conclusion
CROs have become ubiquitous in clinical research amidst the trend toward partnering with third-party providers and the rise of specialized end-to-end clinical development services. A well-equipped CRO will be a valuable ally in managing drug development and clinical trial operations, bringing expertise and experience as well as access to state-of-the-art technology and facilities. Often, partnering with a CRO means optimizing resource allocation and thus keeping costs down. If you’re considering partnering with a CRO to enhance your research, development, or clinical trial efforts, it’s important to make an informed decision to ensure your operations are in the right hands. If you’re looking for a partner specializing in modern patient recruitment, reach out to us – we will be happy to show you the incredible power that lies within our patient recruitment marketplace and advanced recruitment solutions.