Three Biggest Clinical Trial Recruitment Challenges And Strategies To Overcome Them
Clinical trials are critical to improving global health and patient outcomes, and patient participation is the backbone of clinical trials. Sadly, patient recruitment and participation are some of the significant clinical trial recruitment challenges, leading to an 80% delay in all clinical trials. Multiple factors contribute to patient recruitment in clinical trials; for instance, lack of awareness, the complexity of the study, and sociocultural issues. These barriers have greatly impacted patient participation in clinical trials and led to poor or incomplete research.
However, there are several strategies to overcome clinical trial recruitment challenges. Today, we will discuss these challenges in detail and provide implementable solutions to overcome them.
Why Are We Talking About Clinical Trial Recruitment Challenges?
Patient recruitment in clinical trials is not only a problem of current times, but even in the past, the lack of awareness and education led to poor research, recruitment, and engagement challenges. Clinical trial recruitment is a critical step in getting a drug to market. They are research studies that aim to evaluate a biological, surgical, or behavioral intervention.
Currently, clinical research trials are the primary way for researchers to determine if a new treatment, drug, or medical device is safe and effective for public and patient use. A clinical trial for drugs has 3 phases, and each requires patient recruitment and participation.
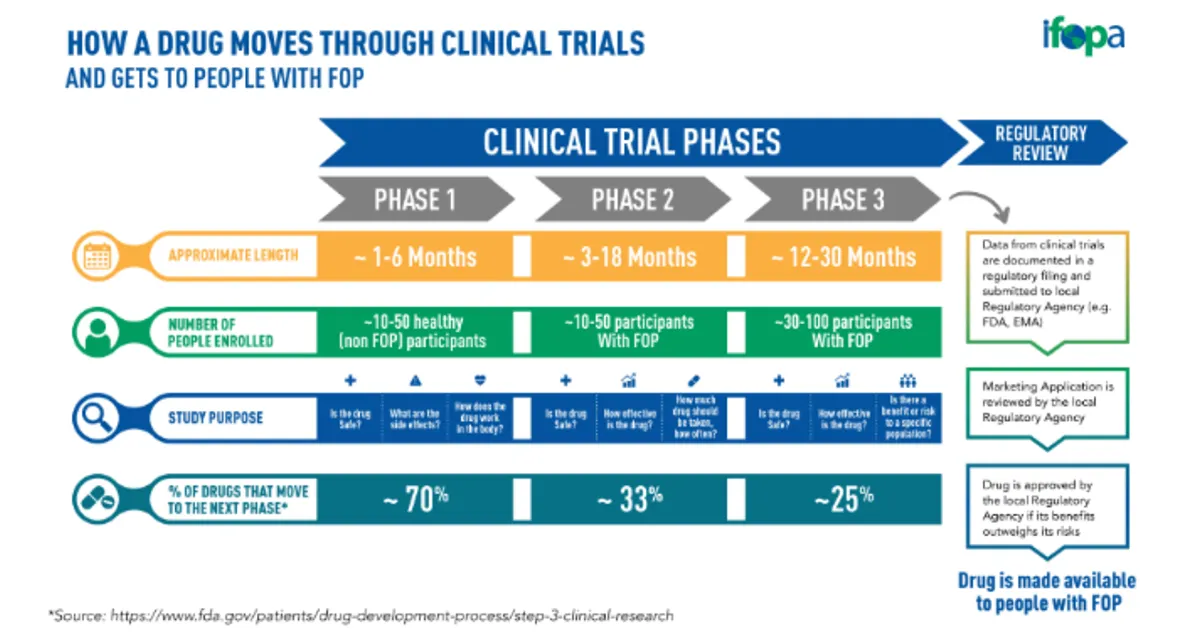
In phase I, investigational drugs start the clinical trial process. In this stage, healthy volunteers are recruited to check whether the drug is safe or has any unwanted side effects. Once cleared, phase II is initiated. A larger group of volunteers are recruited that have the disease. At this phase, additional testing takes place to determine if the drug has the desired effect and what needs to be improved. In phase III, 30 to 100 participants are recruited, and placebo-controlled trials take place to see how patients react to the drug.
Thus, it is apparent that clinical trials will fail without adequate patient participation. Research has shown a clear gap between the willingness of patients to participate in trials and their actual participation rates, which is due to multiple patient-related barriers. For instance, most vendor products are not particularly engaging; patients are unable to offer long-term commitments. Moreover, clinical trials fail to recruit representative participant populations; as a result, only 5% of eligible patients participate in clinical research.
Thus, clinical trial recruitment in the U.S. is a huge barrier to developing new drugs. An analysis shows that a low accrual rate has been the most common termination reason from 2010 to 2021, comprising 25.4% of all terminated trials. Other reasons include financial issues, poor strategic decisions, adverse events, and lack of efficiency.
How Does Patient Recruiting For Clinical Trials Typically Work?
To overcome clinical trial recruitment challenges, you first need to evaluate the current steps CROs or sponsors would take to recruit patients.
The first step of patient recruitment in clinical trials involves study design. Here, you need to dedicate adequate time and resources to enhance patient recruitment and ensure all necessary information is provided so eligible patients can participate.
Next, research centers develop an online advertising database to engage their audience and call patients. Often, clinical research sites are outdated and lagging, which leads to low participation rates. Next, eligibility criteria are set, and the selected patients are called to the clinic for a visit.
The problem with such clinical trials patient recruitment strategy is that patients face structural barriers, such as transportation unavailability, travel cost, too narrow or broad eligibility criteria, presence of comorbid conditions, etc.
Nowadays, clinical trials have considerably evolved and are not only targeted to prestigious people as they used to be. Today, clinical trials are highly structured and regulated and adhere to the highest regulation and ethical compliance standards. Clinical trial recruitment now involves the following steps:
- Clinical led recruitment
- Traditional media
- Social media
- Analytics platform - this involves EHR (electronic health record), an electronic version of a patient’s medical records that the provider maintains and contains clinical data such as progress notes, medications, etc.
Three Biggest Clinical Trial Recruitment Challenges And Strategies To Overcome Them
Clinical trial recruitment challenges are the biggest barriers to systematic study. The lack of awareness and the use of traditional advertising methods have made recruitment a hassle. Still, there are ways to overcome these issues and ensure your study is completed within the time frame.
Following are the 3 biggest clinical trials patient recruitment challenges and implementable strategies to overcome them.
Challenge 1: Traditional recruitment is constrained by the established patient population at research sites
- Only a limited number of doctors participate in clinical research - According to the Food and Drug Administration (FDA) data, only 3% of the nation’s physicians and patients participate in clinical trial research that leads to new therapies. This is because, traditionally, only patients treated at prestigious medical institutions or from medical teams well-connected in the research community were referred for trials. Thus, underserved and nonwhite populations participate at rates less than half of their representation in the general population. When trials are representative, it leads to drug approval that can cause harm or failure in the population.
- Patients outside research sites have difficulty finding clinical trials. Patients who don’t have access to prestigious medical institutions or top doctors face difficulty finding authentic and reliable clinical trials. Such individuals usually browse online, on social media platforms, or by consulting their doctor, but there is no guarantee that the information is trustable.
Strategy: Increase the catchment area and access for patients who aren't seeing research doctors
Having less than half of the eligible representative population in clinical trials harms physicians, patients, and the population worldwide. Thus, clinical trials must develop a dedicated recruitment strategy and use multiple marketing methods to ensure their message reaches the right audience.
Following are some marketing techniques the recruitment team can employ:
- Physician referral networks - widen your reach by connecting with referring physicians. Reach out to local community clinics and prestigious hospitals so your clinical trial population is diverse.
- Inbound patient recruitment platforms - create or use a platform that proactively attracts candidates by providing resourceful information about every trial currently available in the United States. Power is an ideal example of such a platform that offers all information upfront and makes research easy.
- Traditional marketing agencies - take advantage of traditional marketing methods, such as emails, messages, social media marketing, brochures, community outreach programs, etc., to create awareness.
Challenge 2: Structural barriers making it difficult or impossible for patients to join clinical trials
Study availability
Patients face numerous structural barriers that make it challenging to participate in clinical trials. For instance, the absence of a clinical trial at the patient’s site would require them to travel to the clinic, which is not feasible for many.
The team from Fred Hutch, Columbia University Irving Medical Center, and the American Cancer Society Cancer Action Network (ACS CAN) combed through 13 studies spanning 15 years that involved over 8,800 patients. They found that 56% of patients didn't have a trial available at their institution.
Getting to the site
Traveling to the clinic site is also among the top clinical trial recruitment challenges. For instance, 70 percent of potential participants typically live more than two hours from trial sites, and not all can commit to travel and bear the costs every day. Thus, decentralization of clinical trials is critical.
This problem became more apparent after the COVID-19 pandemic when travel became limited by physical distancing and patients’ access to trial sites was reduced by 80%.
Strategy: Leverage technology to reduce logistical friction
Appropriate steps should be taken to decentralize clinical trials and reduce logistical friction. Research centers can utilize technology and make the location a negligible factor in trials. For instance, leverage technology and increase participation through virtual inclusion.
Decentralization will also lessen the workload for investigators and reduce the costs involved by negating the need for a physical location.
Following are the ways you can start leveraging technology:
- Digital site engagement - trial investigators can activate the site through digital channels, such as hiring, engaging, and paying investigators, developing a remote channel, and conducting remote monitoring. You can use social media platforms, forums, and research sites to increase engagement.
- Online patient enrollment - use digital platforms to create awareness about the trial and search for volunteers online. Conduct pre-identification, identification, and eligibility criteria online to save time on-site. You can use online recruitment portals to streamline the process. Ensure that all necessary information is included so patients can easily search.
- Power - Power is an inbound patient recruitment platform that offers cutting-edge research where patients can find currently available clinical trials in the U.S. Power is paving the way for patients by simplifying search by location and offering risk transparency. This platform helps decentralize trials and makes them accessible to everyone.
Challenge 3: Patients' reservations around participating in a trial
Patients worried about participating in a trial - Among the many clinical trial recruitment challenges, the fear of commitment is substantial. Patients are unaware of the purpose or success rate of the study and are fearful about participation. This seed of mistrust was sowed due to the many trials and errors conducted in the past, such as the Tuskegee Syphilis Study or the human experimentation with radiation after WWII.
Patients overwhelmed and confused - Patients participating in clinical trials often feel overwhelmed and confused about the plethora of information being thrust upon them. For instance, balancing physician advice, clinical trial advice, their own research, and being under the family influence can make anyone question their decision, which leads to them dropping out in the middle of the research or refusing to sign up.
According to research, the sheer volume and complexity of information at hand overwhelm patients’ decision-making capacity, and informed consent is, therefore, not possible.
Strategy: Design a patient-centric experience
The only way you can overcome these clinical trial recruitment challenges is by increasing engagement and using a patient-first approach. For instance, the solution lies in making your research interesting and valuable. Although leveraging technology is at the forefront of eliminating these challenges, your focus should be on improving patient experience and using smart tools that decentralize clinical trials. Here is where Power comes in.
As stated above, Power is an inbound patient recruitment platform that enables patients to search clinical trials in the U.S. by location, condition, or drug type. This platform connects patients directly to researchers without having to leave their homes.
Improvements In Patient Recruitment In Clinical Trials Can Unlock Immense Value
Clinical trial recruitment challenges affect a study greatly. Did you know that about 10% of trials fail to enroll even one patient? This is due to structural and educational barriers, lack of online information, and sketchy recruitment platforms. A longer-than-expected recruitment period increases expenses and causes further delays in the drug being introduced in the market.
By leveraging technology and following the above-mentioned strategical approaches, we can decentralize the patient recruitment process and save time, money, and resources. Following is a summary of the points discussed above:
- Researchers can generate interest by empowering patients, sharing information, and including patient feedback in the trial database.
- Utilize inbound recruitment platforms to make research accessible and reliable.
- Utilize social media and other marketing channels to create awareness.
- Create a hybrid model to reduce travel requirements and costs.